| Login | ||

Healthcare Training Institute - Quality Education since 1979
CE for Psychologist, Social Worker, Counselor, & MFT!!

Section 3
Medical Errors: Pre-Analytical Issue in Patient Safety
Question 3
| Test
| Table of Contents
Introduction
Medical error and patient harm have been described and studied for well over a century. However, apart from a few isolated pioneers, the medical and nursing professions did not appear to recognize the extent and seriousness of the problem or, if they did, were not prepared to acknowledge it (1). During the past decade, after the publication of the Institute of Medicine report, To Err Is Human (2), patient safety finally became the object of medical and public attention.
The awareness and understanding of medical errors have expanded rapidly, with an energetic patient safety movement promoting safer health care through "systems" solutions, thanks to a major message from the IOM report: the cause of medical errors and preventable deaths was not careless or incompetent people but bad systems (3).
Compared with other types of medical error, however, diagnostic errors and, in particular, errors in laboratory medicine received little attention and the reasons for this neglect are complex.
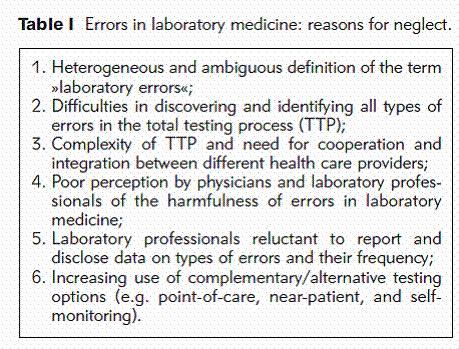
Impact of Errors in Laboratory Medicine
Only a small proportion of laboratory errors results in actual patient harm and adverse events thanks to the several barriers and defensive layers present between the release of laboratory information, the decision-making process and, ultimately, the action on the patient. Data reported in the literature on the impact of laboratory errors on patient care, however, underline that about 25–30% of laboratory errors may have some effects on patient care, while about 6–10% translate into adverse events or risk of adverse events [5, 6, 7, 21].
From a risk management viewpoint, the great majority of laboratory errors with little direct impact on patient care provide important learning opportunities. In fact, any error, regardless of its apparent triviality, might indicate weaknesses in policies and procedures that may not lead to adverse events in their particular context, but might cause the patient harm in slightly different circumstances.
Processes to Reduce Pre-analytical Errors in Laboratory Medicine
In the last few years, in addition to efforts aiming to reduce analytic errors and improve analytic quality, important achievements have been made in addressing errors in laboratory medicine. Thanks to the introduction of pre-analytic workstations, a significant reduction has been achieved in pre-analytic errors due to procedures performed in the laboratory such as specimen preparation through centrifugation, aliquoting, pipetting, and sorting [24, 34]. The increasing interest shown in developing guidelines and standard operating procedures for patient identification, blood collection, sample handling, and specimen acceptance or rejection will surely translate into higher quality standards [25–38]. However, further efforts are needed to translate these initiatives into clinical practice.
The Working Group on "Laboratory Errors and Patient Safety"(WG-LEPS) of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) has undertaken a project named "model of quality indicators" based on the identification of valuable and consensually accepted quality indicators in all steps of the testing process. Briefly, 25 quality indicators were selected after discussing and analyzing the proposal made by 26 clinical laboratories enrolled in the Working Group: 16 for the pre-analytic, 3 for the analytic and 6 for the post-analytic phase. Currently, participant laboratories may introduce the data collected in their own institution on each and all quality indicators in a specifically developed website (www3.centroricercabiomedica.it) [35].
Conclusion
In the last two decades significant advances have been achieved in the comprehension and reduction of errors in medicine. In laboratory medicine, the first lesson we have learned is that the unique framework for identifying and reducing error is TTP, including initial steps such as patient identification and appropriateness in test requesting, and final steps, such as communication and interpretation of test results.
Process analysis, the recording/documentation of all procedures and processes according to quality standards, particularly the ISO 15189: 2007 [36] which has been specifically developed for medical laboratories, are key tools for changing and improving upon every-day clinical practice. The accurate analysis and control of all procedures and processes included in the testing process, particularly if effective tools such as FMEA and HAZOP techniques are adopted, may significantly reduce weaknesses and vulnerable steps, thus maximizing patient safety [37–39].
--Plebani, M., & Piva, E. (2010). MEDICAL ERRORS: PRE-ANALYTICAL ISSUE IN PATIENT SAFETY. Journal Of Medical Biochemistry, 29(4), 310-314. doi:10.2478/v10011-010-0039-2.
Personal
Reflection Exercise #3
The preceding section contained information
about laboratory errors. Write one case study example
regarding how you might use the content of this section in your practice.
Reviewed 2023
Update
Medication errors in community pharmacies:
Evaluation of a standardized safety program
Ledlie, S., Gomes, T., Dolovich, L., Bailey, C., Lallani, S., Frigault, D. S., & Tadrous, M. (2022). Medication errors in community pharmacies: Evaluation of a standardized safety program. Exploratory research in clinical and social pharmacy, 9, 100218. https://doi.org/10.1016/j.rcsop.2022.100218
Peer-Reviewed Journal Article References:
Cipollina, R., & Sanchez, D. T. (2019). Reducing health care disparities through improving trust: An identity safety cues intervention for stigmatized groups. Translational Issues in Psychological Science, 5(4), 315–325.
Ward, M., McAuliffe, E., Fitzsimons, J., & O'Donovan, R. (2019). Informing healthcare team performance: Integrating data to improve quality and safety. International Perspectives in Psychology: Research, Practice, Consultation, 8(1), 53–56.
Zonana, J., Simberlund, J., & Christos, P. (2018). The impact of safety plans in an outpatient clinic. Crisis: The Journal of Crisis Intervention and Suicide Prevention, 39(4), 304–309.
QUESTION 3
What percent of laboratory errors may have some effect on patient care? To select and enter your answer go to Test.
Test
Section 4
Table of Contents
Top
Excerpts from Bibliography referenced in this article
5. Plebani M, Carraro P. Mistakes in a stat laboratory: types and frequency. Clin Chem 1997; 43: 1348–51.
6. Carraro P, Plebani M. Errors in a stat laboratory: types and frequencies 10 years later. Clin Chem 2007; 53: 1338–42.
7. Astion ML, Shojana KG, Hamil TR, Kim S, Ng VL. Classifying laboratory incidents reports to identify problems that jeopardize patient safety. Am J Clin Pathol 2003; 120: 18–26.
21. Bonini P, Plebani M, Ceriotti F, Rubboli F. Errors in laboratory medicine. Clin Chem 2002; 48: 691–8.
24. Da Rin G. Pre-analytical workstations: a tool for reducing laboratory errors. Clin Chim Acta 2009; 404: 68–74.
25. Da Rin G. Pre-analytical workstations as a tool for reducing laboratory errors. Journal of Medical Biochemistry 2010; 29: 315–24.
26. Lippi G, Guidi GC, Mattiuzzi C, Plebani M. Pre-analytical variability: the dark side of the moon in laboratory testing. Clin Chem Lab Med 2006: 44: 358–65.
27. Lippi G, Guidi GC. Risk management in the pre- analytical phase of laboratory testing Clin Chem Lab Med 2007; 45: 720–7.
28. Lippi G, Banfi G, Buttarello M, Ceriotti F, Daves M, Dolci A, Caputo M, Giavarina D, Montagnana M, Miconi V, Milanesi B, Mosca A, Morandini M, Salvagno GL. Recommendations for detection and management of unsuitable samples in clinical laboratories. ClinChem Lab Med 2007; 45: 728–36.
29. Lippi G, Blanckaert N, Bonini P, Green S, Kitchen S, Palicka V, Vassault AJ, Plebani M. Haemolysis: an overview of the leading cause of unsuitable specimens in clinical laboratories. Clin Chem Lab Med 2008; 46:764–72.
30. Lippi G, Salvagno GL, Favaloro EJ, Guidi GC. Survey on the prevalence of hemolytic specimens in an academic hospital according to collection facility: opportunities for quality improvement. Clin Chem Lab Med 2009; 47: 616–8.
31. Lippi G, Salvagno GL, Montagnana M, Brocco G, Guidi GC. Influence of hemolysis on routine clinical chemistry testing. Clin Chem Lab Med 2006; 44: 311–16.
32. Plebani M, Lippi G. Hemolysis index: quality indicator or criterion for sample rejection? Clin Chem Lab Med 2009; 47 (8): 899–902.
33. Lippi G, Luca Salvagno G, Blanckaert N, Giavarina D, Green S, Kitchen S, Palicka V, Vassault AJ, Plebani M.Multicenter evaluation of the hemolysis index in automated clinical chemistry systems. Clin Chem Lab Med 2009; 47(8): 934–9.
34. Holman JW, Mifflin TE, Felder RA, Demers LM. Evaluation of an automatic pre-analytical robotic work - station at two academic health centers. Clin Chem 2002; 48: 540–8.
35. Sciacovelli L, Plebani M. The IFCC working group on laboratory errors and patient safety. Clin Chim Acta 2009; 404: 79–85.
36. ISO 15189: 2007. Medical laboratories – Particular requirements for quality and competence.
37. Chiozza ML, Ponzetti C. FMEA: a model for reducing medical errors. Clin Chim Acta 2009; 404:75–8.
38. Plebani M, Ceriotti F, Messeri G, Ottomano C, Pansini N, Bonini P. Laboratory network of excellence: enhancing patient safety and services effectiveness. Clin Chem Lab Med 2006; 44: 150–60.

