
Healthcare Training Institute
- Quality Education since 1979
CE for Psychologist,
Social Worker, Counselor, & MFT!!

Manual of Articles Sections 1 - 9
Section 3
Medical Errors: Pre-Analytical Issue in Patient Safety
|
| |
Introduction
Medical error and patient harm have been described and studied for well over a century. However, apart from a few isolated pioneers, the medical and nursing professions did not appear to recognize the extent and seriousness of the problem or, if they did, were not prepared to acknowledge it (1). During the past decade, after the publication of the Institute of Medicine report, To Err Is Human (2), patient safety finally became the object of medical and public attention.
The awareness and understanding of medical errors have expanded rapidly, with an energetic patient safety movement promoting safer health care through "systems" solutions, thanks to a major message from the IOM report: the cause of medical errors and preventable deaths was not careless or incompetent people but bad systems (3).
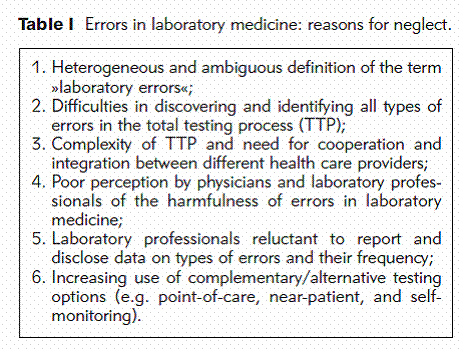
Compared with other types of medical error, however, diagnostic errors and, in particular, errors in laboratory medicine received little attention and the reasons for this neglect are complex.
Impact of Errors in Laboratory Medicine
Only a small proportion of laboratory errors results in actual patient harm and adverse events thanks to the several barriers and defensive layers present between the release of laboratory information, the decision-making process and, ultimately, the action on the patient. Data reported in the literature on the impact of laboratory errors on patient care, however, underline that about 25–30% of laboratory errors may have some effects on patient care, while about 6–10% translate into adverse events or risk of adverse events [5, 6, 7, 21].
From a risk management viewpoint, the great majority of laboratory errors with little direct impact on patient care provide important learning opportunities. In fact, any error, regardless of its apparent triviality, might indicate weaknesses in policies and procedures that may not lead to adverse events in their particular context, but might cause the patient harm in slightly different circumstances.
Processes to Reduce Pre-analytical Errors in Laboratory Medicine
In the last few years, in addition to efforts aiming to reduce analytic errors and improve analytic quality, important achievements have been made in addressing errors in laboratory medicine. Thanks to the introduction of pre-analytic workstations, a significant reduction has been achieved in pre-analytic errors due to procedures performed in the laboratory such as specimen preparation through centrifugation, aliquoting, pipetting, and sorting [24, 34]. The increasing interest shown in developing guidelines and standard operating procedures for patient identification, blood collection, sample handling, and specimen acceptance or rejection will surely translate into higher quality standards [25–38]. However, further efforts are needed to translate these initiatives into clinical practice.
The Working Group on "Laboratory Errors and Patient Safety"(WG-LEPS) of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) has undertaken a project named "model of quality indicators" based on the identification of valuable and consensually accepted quality indicators in all steps of the testing process. Briefly, 25 quality indicators were selected after discussing and analyzing the proposal made by 26 clinical laboratories enrolled in the Working Group: 16 for the pre-analytic, 3 for the analytic and 6 for the post-analytic phase. Currently, participant laboratories may introduce the data collected in their own institution on each and all quality indicators in a specifically developed website (www3.centroricercabiomedica.it) [35].
Conclusion
In the last two decades significant advances have been achieved in the comprehension and reduction of errors in medicine. In laboratory medicine, the first lesson we have learned is that the unique framework for identifying and reducing error is TTP, including initial steps such as patient identification and appropriateness in test requesting, and final steps, such as communication and interpretation of test results.
Process analysis, the recording/documentation of all procedures and processes according to quality standards, particularly the ISO 15189: 2007 [36] which has been specifically developed for medical laboratories, are key tools for changing and improving upon every-day clinical practice. The accurate analysis and control of all procedures and processes included in the testing process, particularly if effective tools such as FMEA and HAZOP techniques are adopted, may significantly reduce weaknesses and vulnerable steps, thus maximising patient safety [37–39].
--Plebani, M., & Piva, E. (2010). MEDICAL ERRORS: PRE-ANALYTICAL ISSUE IN PATIENT SAFETY. Journal Of Medical Biochemistry, 29(4), 310-314. doi:10.2478/v10011-010-0039-2
QUESTION 3
What percent of laboratory errors may have some effect on patient care? Record the letter of the correct answer the
for this course
Forward
to
Back to



